Learning Outcomes
By the end of this lesson, students will be able to:
i. Define and explain the concept of gas pressure, the force exerted by gas molecules against the walls of a container.
ii. Describe the relationship between gas pressure, volume, and temperature, understanding how changes in one affect the others.
iii. Apply Boyle's law, Charles's law, and Gay-Lussac's law to predict and explain the behavior of gases under varying conditions.
iv. Recognize the significance of the ideal gas law, which combines Boyle's law, Charles's law, and Gay-Lussac's law into a single equation.
v. Utilize the gas laws to solve quantitative problems involving gas pressure, volume, and temperature.
Introduction
The world around us is filled with gases, invisible yet ubiquitous, playing a crucial role in our environment and daily lives. Understanding the behavior of gases is essential to comprehending various phenomena, from weather patterns to the functioning of internal combustion engines.
i. Gas Pressure: The Force of the Gaseous Realm
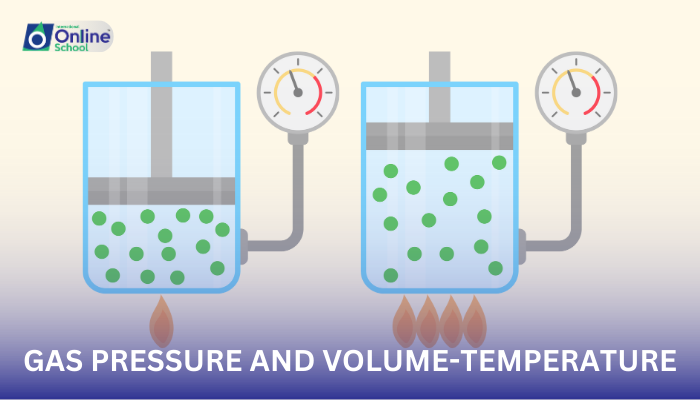
Gas pressure, the force exerted by gas molecules against the walls of a container, is a fundamental property of gases. This pressure arises from the constant motion of gas molecules, which collide with the container walls, generating a force.
ii. Boyle's Law: An Inverse Relationship
Boyle's law, a cornerstone of gas laws, states that at a constant temperature, the pressure of a gas is inversely proportional to its volume. This means that as the volume of a gas decreases, its pressure increases, and vice versa.
iii. Charles's Law: A Warm Embrace
Charles's law, another crucial gas law, states that at a constant pressure, the volume of a gas is directly proportional to its temperature in Kelvin. This implies that as the temperature of a gas increases, its volume expands, and vice versa.
iv. Gay-Lussac's Law: A Hot and Pressurized Affair
Gay-Lussac's law, further elucidating the behavior of gases, states that at a constant volume, the pressure of a gas is directly proportional to its temperature in Kelvin. This means that as the temperature of a gas increases, its pressure rises, and vice versa.
v. The Ideal Gas Law: A Unified Framework
The ideal gas law, a comprehensive equation, combines Boyle's law, Charles's law, and Gay-Lussac's law into a single expression. It relates the pressure, volume, and temperature of an ideal gas, providing a powerful tool for understanding gas behavior under various conditions.
Examples of Gas Laws in Action
Inflating a Balloon: As the volume of a balloon increases when inflated, its pressure decreases, consistent with Boyle's law.
Hot Air Balloons: The increased volume of heated air inside a hot air balloon causes it to rise, demonstrating Charles's law.
Pressure Cookers: The elevated pressure inside a pressure cooker, due to the increased temperature, allows for faster cooking, reflecting Gay-Lussac's law.
The gas laws, Boyle's law, Charles's law, and Gay-Lussac's law, collectively represented by the ideal gas law, provide valuable insights into the behavior of gases under varying conditions. By understanding these relationships, we gain a deeper appreciation for the delicate balance between pressure, volume, and temperature in the gaseous world.